China bio firm dispels fears of serious blood clots from its COVID-19 vaccine

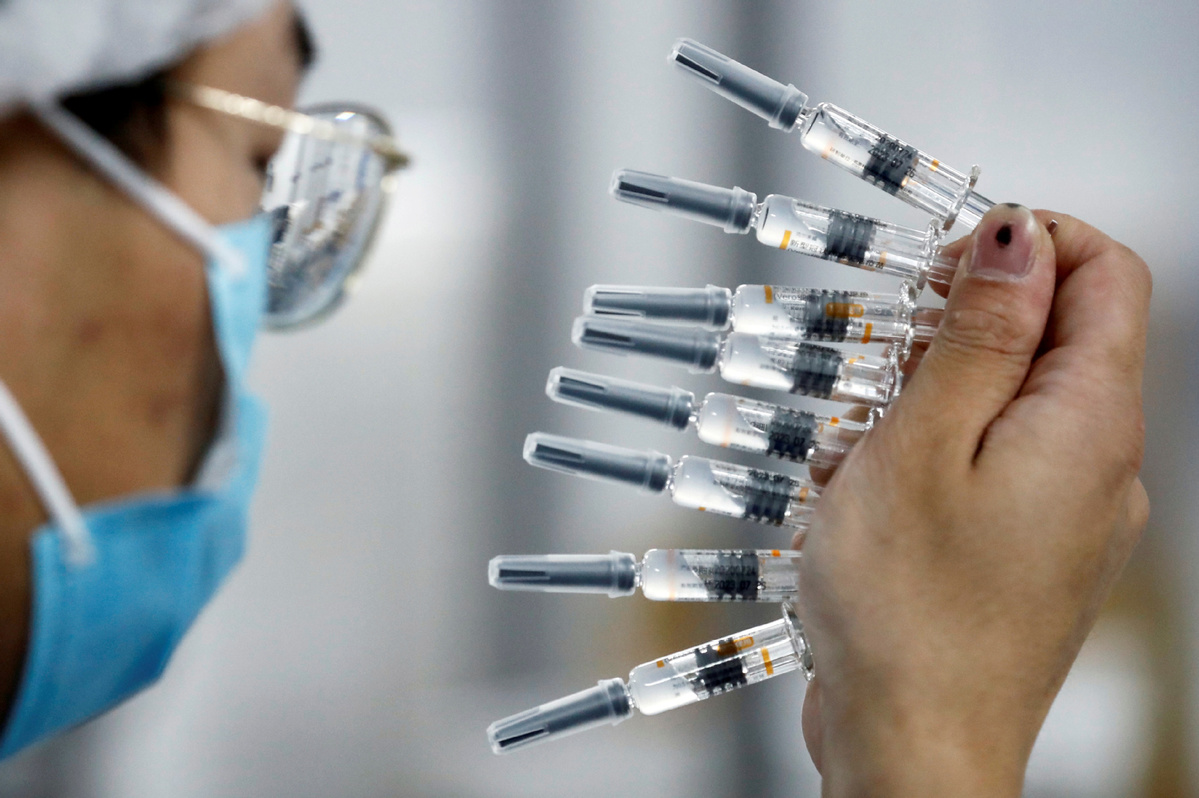
BEIJING -- China's CanSino Biologics Inc (CanSinoBIO) has said that no serious blood clot cases were reported in the recipients of its COVID-19 vaccine Ad5-nCoV.
"No blood clot related serious adverse events have been reported in around one million vaccinations of Ad5-nCoV," CanSinoBIO said in a statement on Wednesday.
The statement came after US federal health agencies on Tuesday called for a pause of the use of Johnson & Johnson's COVID-19 vaccine, which applies a similar technology to Ad5-nCoV, following some blood clots reports.
Although both vaccines developed by CanSinoBIO and Johnson & Johnson's contained another virus called adenovirus, CanSinoBIO's vaccine uses a different adenovirus from that for Johnson & Johnson's.
In mid-January, Russian pharma Petrovax said that 92.5 percent of Russian volunteers in trials of the Ad5-nCoV vaccine had shown high levels of antibodies, according to Russia's Interfax news agency.
So far, the Ad5-nCoV vaccine has been approved for emergency use in China, Hungary, Chile, Mexico and Pakistan.
- Pioneering deep-sea mission completed
- Foreigners back Xizang's boarding school system
- Compatriots from both sides of Taiwan Strait oppose external interference
- Ex-deputy GM of key state-owned enterprise expelled from CPC for corruption
- Legislators push stronger protections for disabled
- Former senior legislator dismissed from public office for duty-related crimes